
Participants were healthy, nondiabetic children and adults, primarily recruited from family, friends, and neighbors of patients seen in the diabetes clinics. Study participants provided written informed consent prior to study participation. The study was conducted at 12 diabetes centers within the T1D Exchange Clinic Network after approval by institutional review boards ( 8). This study was undertaken to provide an updated set of normative CGM sensor glucose data in a large group of healthy, nondiabetic children, adolescents, and adults. Most studies of CGM profiles in healthy, nondiabetic individuals have had small sample sizes or used early-generation CGM systems, which were less accurate than current devices ( 3–7). However, there are limited data on CGM-measured glucose concentrations in individuals without diabetes. Understanding CGM metrics in healthy participants without diabetes is needed to serve as a benchmark for research studies to define impaired glycemic status and for future technologies aiming to help individuals achieve near-normal glycemic profiles. Moreover, CGM metrics are being used to examine and compare the relative effectiveness and safety of new therapeutic agents and modern technologies in clinical diabetes research. With currently available accurate CGM devices, management of patients with type 1 diabetes (T1D) has increasingly depended on CGM for day-to-day adjustments of diabetes treatment ( 2).

However, HbA1c does not capture clinically relevant hyperglycemic and hypoglycemic patterns, which limits its utility in personalizing insulin-dosing decisions.Ĭontinuous glucose monitoring (CGM) provides information on trends for hypoglycemia, hyperglycemia, and glycemic variability that helps patients and providers optimize glycemic control without increasing the risk for hypoglycemia. This is due to the fact that HbA1c is a reflection of overall glycemic control from the prior 60-90 days, as well as clear evidence from the DCCT establishing it as a surrogate marker for diabetes microvascular complications risk ( 1).
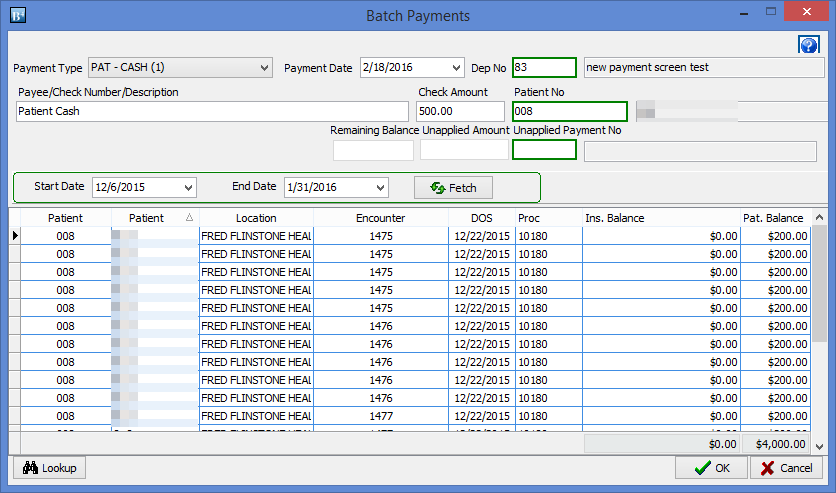
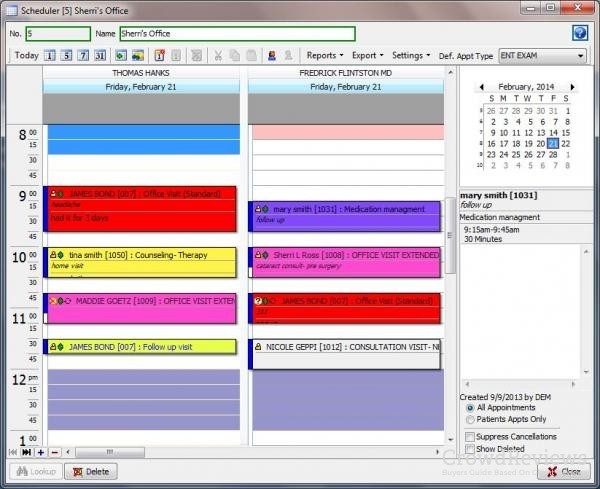
CGM DAQBILLING 4.0.21.12 UPDATE TRIAL
Since the publication of the Diabetes Control and Complication Trial results in 1993, glycated Hb (HbA1c) has been the gold standard to assess outcomes of diabetes management.


 0 kommentar(er)
0 kommentar(er)
